Shan et al. (Acta Pharm. Sin. B., 2022) have summarized the industry interest in nanotechnology for drug delivery based on registered clinical trial data from the NIH. Liposome formulations account for 33% of clinicals trials with polymeric formulations accounting for 20%. The data suggests there is merit for each type of formulation for specific applications.
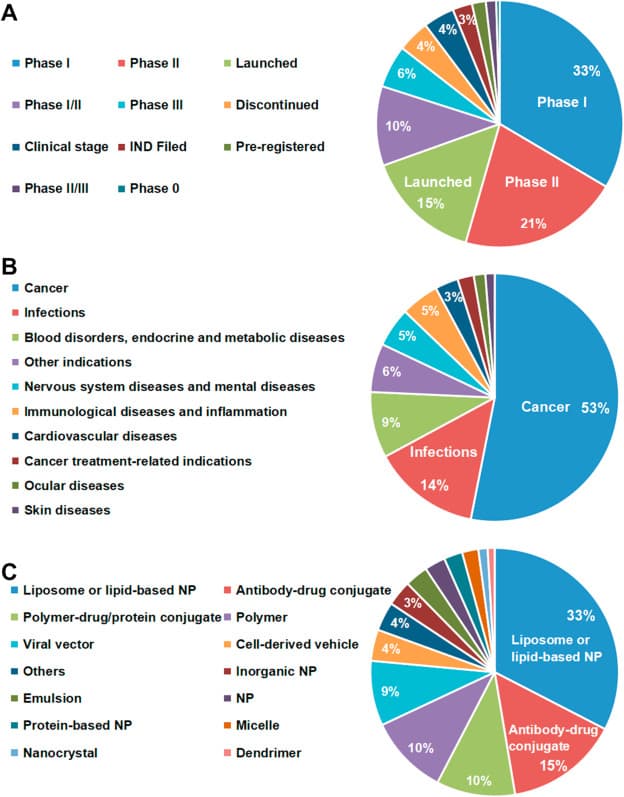
Acta Pharm. Sin. B.2022https://doi.org/10.1016/j.apsb.2022.02.025
Interest in applying nanoparticles for various therapeutic fields continues to grow year over year in academic publications since the year 2000; reflecting industry interest. Oncology/cancer, hematology, immunology, and inflammation remains the top therapeutic fields to pioneer drug delivery with nanotechnology among scientists.
Loading plot...
Applying Nanotechnology for Cancer
The approval rate of cancer therapeutics remains less than 10%, marred by various challenges such as high risk-to-benefit, toxicity, and lack of efficacy. Our review of NIH 253 cancer clinical trials between 2000 – 2022 using nanotechnology indicate 11% of Phase 2 trials progress to Phase 3 compared to 28% without the use of nanotechnology. The data suggests the field of nanotechnology is missing insight into translating nanoparticles as therapeutic vehicles for cancer drugs.
Loading plot...
Academic research has demonstrated materials such as PLGA, chitosan, gelatin, lipids, etc. are non-toxic for therapeutic applications. This is only part of the equation for a successful therapeutic formulation. For formulations without targeting ligands (e.x. antibodies, peptides, etc.) these formulations may be modified for longer systemic circulation time rather than site-directed targeted delivery. In which case, the following questions remains:
a) Does the encapsulation of therapeutics within nanoparticles increase or decrease efficacy?
b) Do the adverse effects remain even when encapsulated?
Our understanding of the in vivo interactions of nanoparticles with the human body remains poorly understood. These macro level interactions are key to identifying the characteristics most suitable for a drug formulation. One solution is to aggregate and analyze the readily available data from the scientific community to draw insights from empirical data.
Targeted delivery of therapeutics remains the holy grail of drug delivery to:
a) Reduce the dosage of a drug given; and
b) Directs the drug to where it is needed most (site-specific drug delivery).
As a result, this increases the efficacy and reduces the side effects of therapies by limiting where the drug goes.
The nanoML platform makes the design of tailored nanoparticle formulations for targeted delivery available to address these challenges. Using an in silico method of design and testing informed by the combined knowledge of the scientific community, the nanoML significantly reduces the pre-clinical R&D time to get a formulation ready for testing.